what is difference between enthalpy and entropy
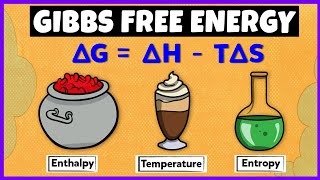
Enthalpy vs. Entropy: What’s the Difference? ❄
In this video, we break down the key differences between enthalpy and entropy, two essential concepts in thermodynamics. Whether you're a student trying to understand these terms or just curious about how energy and disorder play a role in physical processes, this video will help clarify it all!
What You’ll Learn:
Enthalpy (H): Learn how enthalpy measures the total heat content of a system and its role in chemical reactions.
Entropy (S): Understand how entropy represents the degree of disorder or randomness in a system.
Key Differences: We’ll compare their definitions, roles in energy exchanges, and how they influence processes like phase changes and chemical reactions. ⚖
If you’re ready to finally grasp the concepts of enthalpy vs. entropy, don’t miss this clear and concise explanation!
Don’t forget to like, subscribe, and hit the notification bell for more science content!
#Enthalpy #Entropy #Thermodynamics #Energy #Physics #Chemistry #ScienceExplained #LearnWithUs